
Aug 29, 2018
Published on ScienceDaily.com
Four out of ten patients with atrial fibrillation but no history of stroke or transient ischaemic attack have previously unknown brain damage, according to the first results of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF) presented today at ESC Congress 2018.
“Our results suggest that clinically unrecognised brain damage may explain the association between dementia and atrial fibrillation in patients without prior stroke,” said Co-Principal Investigator Professor David Conen of McMaster University, Hamilton, Canada.
Patients with atrial fibrillation have a significantly increased risk of stroke, which is why most are treated with blood thinners (oral anticoagulation). This increased stroke risk is probably the main reason why patients with atrial fibrillation also face an increased risk of cognitive dysfunction and dementia. However, the relationship between atrial fibrillation and dementia has also been shown among patients without prior strokes, meaning that additional mechanisms have to be involved.
Clarifying the mechanisms by which atrial fibrillation increases the risk of cognitive dysfunction and dementia is a first step towards developing preventive measures.
Swiss-AF is a prospective, observational study designed to pinpoint the mechanisms of cognitive decline in patients with atrial fibrillation.2 This analysis investigated the prevalence of silent brain damage in atrial fibrillation patients.
The study enrolled 2,415 patients aged over 65 years with atrial fibrillation between 2014 and 2017 from 14 centres in Switzerland. All patients without contraindications underwent standardised brain magnetic resonance imaging and the images were analysed in a central core laboratory. Scans were available in 1,736 patients. Of those, 347 (20%) patients had a history of stroke and/or transient ischaemic attack and were excluded from the analysis.
The final analysis included 1,389 patients with atrial fibrillation but no history of stroke or transient ischaemic attack. The average age of participants was 72 years, and 26% were women. The scans showed that 569 (41%) patients had at least one type of previously unknown brain damage: 207 (15%) had a cerebral infarct, 269 (19%) had small bleeds in the brain (microbleeds), and 222 (16%) had small deep brain lesions called lacunes.
“Four in ten patients with atrial fibrillation but no history of stroke or transient ischaemic attack had clinically unrecognised ‘silent’ brain lesions,” said Professor Conen. “This brain damage could trigger cognitive decline.”
Most study participants (1,234; 89%) were treated with oral anticoagulants. Co-Principal investigator Professor Stefan Osswald of University Hospital Basel, Switzerland, noted that the cross-sectional analysis looked at the data at a single point in time and cannot address the question of whether the cerebral infarcts and other brain lesions occurred before or after initiation of oral anticoagulation. But he said: “The findings nevertheless raise the issue that oral anticoagulation might not prevent all brain damage in patients with atrial fibrillation.”
Professor Conen said: “All Swiss-AF participants underwent extensive cognitive testing. These data will be analysed to see whether patients with silent brain lesions also have impaired cognitive function.” Collaborations with other study groups will help to sort out whether these findings are specific to patients with atrial fibrillation.
Story Source:”Four out of 10 patients with atrial fibrillation have unknown brain damage.” ScienceDaily. ScienceDaily, 26 August 2018. <www.sciencedaily.com/releases/2018/08/180826120744.htm>.

Aug 28, 2018
The original information provided at Braincouncil.eu
In July this year, European Brain Council launched a statement “Counting down to zero: Towards a future with underfunded health research?” which calls on the European Commission, the European Parliament and the Council to increase the budget of the “Horizon Europe” programme to at least €120 billion. What is more, the statement calls on European decision-makers to redistribute the budget in order to ensure that more funding is allocated to the “health” cluster under Pillar II.
The proposal for “Horizon Europe”, published in June 2018, includes an overall budget of €94.1 billion in inflation-adjusted prices. Within this budget, roughly €7.7 billion is proposed to fund the “Health” cluster that is part of the pillar “Global Challenges and Industrial Competitiveness”.
EBC welcomes the increase of the overall draft budget but firmly believes that the proposed budget as it currently stands is insufficient to effectively address today’s societal challenges. The treatment of brain disorders alone is estimated to cost close to €800 billion annually[1], which makes adopting a robust 9th Framework Programme with a strong focus on accelerating health research of paramount importance. What is more, the proposed budget for the “Health” cluster confirms a steady decrease of funding over time and across Framework Programmes, as health was previously allocated 12% under the 7th Framework Programme, 10% under Horizon 2020 and now 8% in the Horizon Europe proposal.
“We are highly concerned about the budget of €7.7 billion provisionally allocated to the “health” cluster under
Pillar II. This amount is not commensurate with the total budget increase and will clearly be insufficient to
effectively address the societal challenges associated with health research. Moreover, this budget confirms a
steady decrease of funding over time and across Framework Programmes, as health was previously allocated
12% under the 7th Framework Programme, 10% under Horizon 2020 and now 8% in the Horizon Europe
proposal. For continued success in European research, we find it imperative that this downward trend is
stopped and reverted.”– says the statement.
The full statement can be accessed here.
EBC invites all organizations, operating at national and/or EU level, that are supportive of the call to join as co-signatory of the statement. Please contact the EBC office (info@braincouncil.eu or +32 (0)2 513 27 57) should you be interested in having the logo of your organization added to the list of signatories.


Aug 24, 2018
The following content was first published on EU Commission official website
According to a recently published study, European patients are still generally unaware of their rights and the possibility to access health services in other EU Member States, as well as of the existence of National Contact Points (NCPs). But the situation is improving.
National Contact Points (NCPs) aim to help patients exercise their rights under the Cross-border Healthcare Directive. But how can they improve their work?
Using a combination of research methods, including a literature review, an analysis of legal texts, a website analysis, a pseudo-patient investigation, and surveys of NCPs and patients, the aim of the study carried out by Ecorys, KU Leuven and GfK Belgium was to identify how to improve the current level of information on cross-border healthcare available to patients.
Websites
The study found that although the information available to patients on NCP websites was adequate, the websites themselves need improvements, especially the sections on patients’ rights (for incoming patients), quality and safety standards (for incoming patients) and reimbursement of cross-border healthcare costs (for outgoing patients).
However, compared to the results of the earlier Evaluative study(fieldwork carried out in 2014), the NCPs have made significant progress in this area.
Toolbox and training material
This study has also resulted in the development of a practice-orientated toolbox and training material to help the NCPs improve the quality of information for patients, as well as a set of Guiding Principles and indicators for establishing an NCP service that is more uniform, patient-centred and in line with the legal requirements. This will contribute to high level information provision to patients.
The study feeds into the upcoming implementation report on the operation of the Cross-border Healthcare Directive due this October.
More information

Aug 8, 2018
Solitaire™ With the Intention For Thrombectomy Plus Intravenous t-PA Versus DIRECT Solitaire™ Stent-retriever Thrombectomy in Acute Anterior Circulation Stroke (SWIFT DIRECT)
Background
The SWIFT DIRECT trial investigates the emergency treatment of patients with an acute ischaemic stroke. An ischaemic stroke is caused by the blockage of one or more blood vessels in the brain. A clot blocks the blood vessels and blood can no longer circulate. This results in an undersupply of blood and oxygen to the brain regions supplied by these vessels. If the under-supply lasts longer than a few minutes, there is a risk that nerve cells might die. An ischaemic cerebral infarction is a life-threatening situation.
For years, the only causal treatment was the administration of a clot-lysing drug called tissue plasminogen activator. The drug, however, may induce bleedings and is not sufficiently effective in patients with very large clots. In 2014, a new treatment, the so-called mechanical thrombectomy, has been established. With this therapy, nearly all types of large blood clots can directly be removed from the vessel using a specialized catheter. Trials have shown that patients treated with tissue plasminogen activator and mechanical thrombectomy have better outcomes than patients treated with tissue plasminogen activator only. Hence, the current standard treatment in patients with large clots is administration of tissue plasminogen activator followed by mechanical thrombectomy.
As administration of tissue plasminogen activator may also harm the patient and is not effective in patients with large clots, we want to investigate how potent direct mechanical thrombectomy (without prior administration of tissue plasminogen activator) is. The purpose of this trial is thus to compare direct removal of the clot with mechanical thrombectomy versus tissue plasminogen activator administration followed by removal of the clot with mechanical thrombectomy. Only patients with large clots and direct access to mechanical thrombectomy can be included in the trial.
We are conducting this trial to improve the emergency treatment for affected patients with an acute ischaemic stroke. This project is organised by the Neuro Clinical Trial Unit at the University of Bern, Switzerland and will be carried out at several hospitals in Europe and Canada.
What does it mean for patients to participate in this clinical trial?
Trial participants will be assigned by chance to one of two groups (half of the patients will be in each group). In the ‘treatment group’ the blood clot is removed directly with mechanical thrombectomy. In the ‘standard group’, participants first receive blood clot-dissolving medication followed by mechanical thrombectomy to remove the clot.
Both treatment options are commonly used standard treatments. The choice between the two is part of clinical routine at the hospital and lies upon the judgement of the treating physician. Except for the phone interview 90 days after the infarction incident, all examinations are part of standard treatment routine independent from the trial.
General information about the trial
| Study type: |
Multicenter, prospective, randomized, open label, blinded endpoint (PROBE) trial |
| Trial start and end: |
October 2017 to December 2020 |
| Sponsor-Investigators: |
Prof. Dr. med. Urs Fischer, Neurology, and
Prof. Dr. med. Jan Gralla, Neuroradiology,
University Hospital Bern, Inselspital, Switzerland |
| Total number of participants: |
404 patients |
| Trial duration for each participant: |
3 months |
| Participating countries: |
Austria, Canada, Finland, France, Germany, Spain and Switzerland |
| Financial support: |
Medtronic (Minneapolis, USA) |
| Trial registration: |
www.clinicaltrials.gov, No.: NCT03192332 |
Please visit the website www.swift-direct.com for further information.
This trial is endorsed by SAFE.

Aug 8, 2018
Swiss trial of decompressive craniectomy versus best medical treatment of spontaneous supratentorial intracerebral hemorrhage (SWITCH): a randomized controlled trial
Background
The SWITCH trial investigates the treatment of patients with spontaneous intracerebral hemorrhage (bleeding in the brain). Bleeding in the brain leads to severe brain dysfunctions due to the hemorrhage itself, but also from the brain swelling (brain edema). Each year, about 2 million people worldwide are affected by this disease. The majority of surviving patients remains handicapped. Apart from the standard best medical treatment, there is no possibility to help these patients until now.
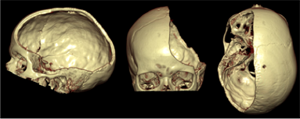 Decompressive craniectomy (removal of parts of the cranial bone) is a standard surgical treatment, which is beneficial in patients with brain swelling after a severe ischemic stroke (under- supply of blood and oxygen in certain brain regions due to occlusion of a blood vessel by a blood clot), after brain injuries, but also in patients with meningitis. However, no trial has so far investigated the effectiveness of decompressive craniectomy in patients with brain bleeding. With this trial we would like to investigate in patients with a brain bleeding, whether this treatment method can reduce mortality and dependency compared to best medical treatment. In a larger context, this trial aims to offer a future treatment option to patients with brain bleeding, which can reduce both mortality and disability.
Decompressive craniectomy (removal of parts of the cranial bone) is a standard surgical treatment, which is beneficial in patients with brain swelling after a severe ischemic stroke (under- supply of blood and oxygen in certain brain regions due to occlusion of a blood vessel by a blood clot), after brain injuries, but also in patients with meningitis. However, no trial has so far investigated the effectiveness of decompressive craniectomy in patients with brain bleeding. With this trial we would like to investigate in patients with a brain bleeding, whether this treatment method can reduce mortality and dependency compared to best medical treatment. In a larger context, this trial aims to offer a future treatment option to patients with brain bleeding, which can reduce both mortality and disability.
This project is organized by the Neuro Clinical Trial Unit at the University of Bern, Switzerland, and is carried out throughout Europe.
What does it mean for patients to participate in this clinical trial?
All patients will be will be assigned by chance to two groups (half of patients will be in each group). In the ‘treatment group’, surgery (decompressive craniectomy) plus best medical treatment will be performed. The patient’s own cranial bone will be re-inserted (re-implanted) after reduction of the swelling in the brain. This usually takes place after about 3 months. In the ‘standard group’, the participants receive best medical treatment, which is the current standard treatment.
A telephone interview will be performed with all patients 30 days, 6 and 12 months after randomization. Apart from the telephone interviews, all examinations are carried out regardless whether or not the patient participates in this trial.
General information about the trial
| Study type: |
Multicenter randomized (1:1) controlled parallel group trial
|
| Trial start and end: |
October 2014 to September 2020
|
| Sponsor-Investigator: |
Prof. Dr. med. Urs Fischer, Inselspital Bern, Switzerland
|
| Participant countries: |
Switzerland, Austria, Germany, Helsinki, Spain, the Netherlands, France
|
| Upcoming participant countries: |
UK
|
| Total Number of participants: |
300 patients |
| Trial duration for each participant: |
12 months |
| Financial support: |
Swiss National Science Foundation (SNCF), Swiss Heart Foundation, Inselspital Foundation |
| Trial registration: |
www.clinicaltrials.gov, No.: NCT02258919 |
For further information, please visit the SWITCH Website
This project is endorsed by SAFE.
Aug 6, 2018
Oruen – The CNS Journal is a peer-reviewed, open access publication, and has received CME accreditation from the European Accreditation Committee in CNS (EACIC), with a 100% focus on original CNS research topics, and the latest advances, diagnoses, and treatment of CNS disorders.
The Journal is distributed in print and electronically to thousands of physicians, researchers, academics, nurses, and related healthcare professionals with an interest in CNS disorders. Both subscription and access are free and there are no contributory author fees for publication. Papers submitted for publication are accepted based on their originality, likely impact on and relevance to clinical practice, data quality, and overall potential interest to the journal’s readership.
Oruen – The CNS Journal is published bi-annually. The first issue of the journal was published in May 2015
You can access the latest issue by clicking on the photo below:
For any questions or submission requests/enquiries please contact Dr James Coe – Head Editor editor@oruen.com
Aug 6, 2018
Early versus Late initiation of direct oral Anticoagulants in post-ischaemic stroke patients with atrial fibrillatioN (ELAN) started with the recruitment in October 2017 with currently approximately 80 sites in Switzerland and European countries.
This pragmatic investigator-initiated international trial will add evidence to the best time of starting DOAC after ischaemic stroke in patients with atrial fibrillation. If earlier initiation of DOACs in patients with ischaemic stroke related to atrial fibrillation is shown to be safe and efficacious, this could have a major impact on better treatment adherence, length of hospital stay and patient outcome.
We used an opportunity to talk with Urs Fischer, Professor for Acute Neurology and Stroke (Extraordinarius) at the University Hospital (Inselspital), Bern and principal investigator of the ELAN trial (www.elan-trial.ch).
1. What is ‘atrial fibrillation’ or ‘AF’ and why is it important to know more about it?
Atrial fibrillation is a condition of the heart that is characterized by an abnormal heart rhythm. If you suffer from AF, the atria, two of the chambers in the heart, beat very rapid and irregular. Atrial fibrillation can be episodic or chronic and symptomatic or asymptomatic. It is a very relevant condition because people with atrial fibrillation have a higher risk of suffering from a stroke or a heart failure. Atrial fibrillation is very widespread around the world, especially in the elderly population and causes major mortality and morbidity.
2. What is your personal involvement with the subject of AF, how long are you interested in this subject?
I am currently working as a Professor for Acute Neurology and Stroke at the University Hospital Bern, Switzerland and I am very passionate to find new approaches to prevent and treat strokes. Stroke has a major impact on the quality of life of my patients and their relatives and one of the main causes of a stroke is AF. Therefore, AF needs to be detected and treated.
3. Is there a way to discover AF in time to prevent stroke?
Yes, AF can be detected by cardiac monitoring. Some patients complain about shortness of breath or an irregular pulse. However, it is not uncommon that atrial fibrillation is only diagnosed after the patient has suffered a stroke, especially when AF is asymptomatic and only occurring in episodes and not all the time. Missing these patients is a problem and improving the diagnosis in such patients is a challenge that occupies physicians around the world.
4. Is AF treatable and how?
Atrial fibrillation can be treated or rather managed in a number of ways. The most important approach in the management of AF is to prevent the formation of clots in the heart, which then can cause a stroke or an embolism in other parts of the body. If a patient with AF is at risk of suffering a stroke, anticoagulants (i.e. blood thinners) should be started by the treating physician. If atrial fibrillation is symptomatic by i.e. shortness of breath, cardiologists try to restore a normal heart rhythm. This can be achieved either with drugs or with (minimal invasive) surgery. If the patient is asymptomatic, anticoagulation and frequency control in case of tachycardia are the main goals of therapy.
5. In your opinion, why is the initiation of direct oral Anticoagulants in post-ischaemic stroke patients with atrial fibrillation important?
Post-ischaemic stroke patients are at a high risk of suffering a second stroke soon after the first event. This is called a recurrent stroke. Treatment with oral anticoagulants reduces the risk of a recurrent stroke significantly. Direct oral anticoagulants are the newest class of anticoagulants and compared to older ones, they are just as effective but safer. However, the time point when these drugs should be started after a stroke is unknown. Therefore, we have designed the ELAN trial (Early versus Late initiation of direct oral Anticoagulants in post-ischaemic stroke patients with atrial fibrillatioN (ELAN): an international, multicentre, randomised-controlled, two-arm, assessor-blinded trial; www.elan-trial.ch), in which we currently assess, whether anticoagulants should be started early or with a certain time delay after a stroke.
6. When did the research project ELAN started and what do you expect would change once the research is completed?
The first hospitals started recruiting patients in October 2017. After completion, the results of this trial will help us to understand more about the safety and the potential benefits of an early treatment start with direct oral anticoagulants compared to a later treatment start. The results will give physicians a scientific justification of when to start treatment. ELAN could possibly change the way how post-ischaemic stroke patients with atrial fibrillation are treated.
7. Who is funding this research and do you involve patients/ stroke support organisations in it?
ELAN is funded by the Swiss National Science Foundation and the Swiss heart Foundation. Furthermore, it is endorsed by SAFE, the Stroke Alliance for Europe.
About Prof. Urs Fischer
Urs Fischer is Professor for Acute Neurology and Stroke (Extraordinarius) at the University Hospital (Inselspital), Bern. He is chair of the Neurological Inpatient Department, co-chair of the Stroke Center Bern, and deputy director of the Clinical Trial Unit (CTU) of the University of Bern. He is a clinical researcher and his main research interest involves diagnosis, treatment and outcome of patients with acute neurological diseases. He is co-principal investigator of the SWITCH study (www.switch-trial.ch), principal investigator of the ELAN trial (www.elan-trial.ch) and co-principal investigator of the SWIFT DIRECT trial (www.swift-direct.ch). Urs Fischer is Secretary General of the European Stroke Organisation (ESO), treasurer of the Swiss Neurological Society (SNG) and co-founder the ESO ESMINT ESNR Stroke Winter School, which is regularly held at the University Hospital in Bern, Switzerland.

Aug 2, 2018
The role of a helpline is to provide information and support in a trusted space through non face-to-face channels. A helpline can provide support throughout the country or in a local area. Helpline practice is needed to create a service remit and boundaries which sets out a clear definition of what the helpline does and doesn’t do. Good helpline practice can involve developing a set of guidelines around how the helpline works on a daily basis.
The Stroke Association Helpline provides information, guidance and support to anyone affected by stroke over the telephone, by e-mail, letter and through social media. On the helpline, we provide enquirers with information about all topics relating to stroke, from what a stroke is to what should happen after a stroke, from stroke prevention to stroke recovery. We will listen, provide reassurance, offer encouragement, make suggestions and signpost to stroke clubs and a range of local and national services and organisations for further support or specific assistance or advice.
Staff working on the Stroke Association Helpline receive weekly debrief sessions which provides a space away from the helpline to talk through any enquiries that may be challenging or emotional. Within the Stroke Association, staff can also access an employee assistance programme which is a confidential support telephone service.
To help us make this hour long webinar on helpline practice is as useful and interesting to you as possible, we would like to hear from you about what you would like us to focus on. Do not worry if you cannot attend the webinar, we will record it and share it with you. Please download the survey questionnaire here and return it to Sarah Belson sarah.belson@stroke.org.uk by 10th September.
Date for the webinar: 25th September
(The time of the webinar will be confirmed once we know where in the world participants are joining from to ensure the best time possible.)
Jul 30, 2018
Brussels, July 30, 2018- The eLearning Module 3 of the Stroke Support Organisation Faculty Tool (SSOFT) is published today at the following address www.ssoft.info.
SSOFT’s third module focuses on understanding different types of evidence; how to collate it and how Stroke Support Organisations can use it to their advantage:
3.1 – Use of evidence
3.2 – Types of evidence
3.3 – Interpreting evidence & data
3.4 – How SSOs generate data & evidence
3.5 – Using evidence & data in your communications
Stroke Support Organisations have been at the heart of this tool. For newer or smaller organisations, the information in the tool will provide knowledge that will help them to build and grow their communities.
Larger organisations can also benefit from SSOFT, which can enable them to support their communities and other stroke professionals across Europe, adding more voices to their movement/arguments for change.
This tool is also for anyone who is interested in knowing more about what an SSO is, how to start and develop one and how to make it sustainable.
For those interested in using this innovative eLearning platform, we would encourage them to visit the SSOFT website www.ssoft.info
About SSOFT
SSOFT is an innovative online eLearning advocacy tool being developed by Stroke Alliance for Europe (SAFE), in partnership with the European Stroke Organisation (ESO).
This online learning platform provides knowledge and training on how the creation of effective advocacy activities and campaigns to deliver positive change at a local and national level on stroke prevention, treatment and care. The eLearning platform will include six modules that provide information on:
Module 1: Stroke Support Organisations (SSOs)
Module 2: Making Change Happen
Module 3: Use of Evidence
Module 4: Role of Patient Voice
Module 5: Health System Advocacy
Module 6: Public Advocacy
The modules and learning environment is accessible via the SSOFT website through a simple registration process. Visitors to the website can also learn more about SSOFT, SAFE and ESO, find their nearest SAFE Stroke Support Organisation (SSO) as well as hear from SAFE members about their experiences.
For more information, please send an email ssoft@safestroke.eu or visit www.ssoft.info
Acknowledgments
SAFE would like to take this opportunity to thank and acknowledge the contributions made by those who have helped in the development of SSOFT and module 1.
Stroke Alliance for Europe Board, who have been involved at every stage of development of this module.
The Peer Reviewers for module 3:
- Stiftung Deutsche Schlaganfall-Hilfe (Dr Markus Wagner)
- Hellenic Alliance/Action for Stroke Support Organization (Dr Hariklia Proios)
- Macedonian Stroke Association (Dr Anita Arsovska & Dr. Maja Bozinovska Smiceska)
- Norsk forening for slagrammede (Ms Grethe Lunde)
- Thessaloniki and Wiesbaden NRZ, Rehabilitation Center Germany (Dr Dimitris Artemis)
- Aristotle University, Greece (Dr Katerina Nicolaidis)
- Anagenissis Rehabilitation Center, Greece (Ms Eugenia Stamatiou)
Our members who have shared their experiences and knowledge in the video interviews used within the module:
- Chris Macey – The Irish Heart Foundation, R.Ireland
- Pnina Rosenzweig – Neeman Association for Stroke Survivors, Israel
- Ove Puisto – STROKE-Riksförbundet, Sweden
- Markus Wagner – Stiftung Deutsche Schlaganfall-Hilfe, Germany
- Monique Lindhout – Hersenletsel, Netherlands
Our partner organisations who have collaborated in the development of the module content:
- World Stroke Organization
- European Stroke Organisation.
And all those who participated in the User Acceptance Testing of Module 3.
We would also like to thank the project sponsor Bayer Healthcare who have supported this project through an education grant.
About SAFE
The Stroke Alliance for Europe (SAFE) a non-profit-making organisation formed in 2004. It is the voice of stroke patients in Europe, representing a range of patient groups from 30 European countries.
SAFE’s goal is to decrease the number of strokes in Europe by advocating for better prevention, access to adequate treatment, post-stroke care and rehabilitation.
For more information about SAFE, please visit www.safestroke.eu

Jul 27, 2018
The 15 min round table discussion by Christina Sjöstrand, Stockholm, Hans-Christoph Diener, Essen and George Ntaios, Thessaly, focuses on the concepts of randomized clinical trials for secondary stroke prevention in patients with embolic stroke of undetermined source (ESUS). Two large clinical trials tested the hypothesis that non-vitamin K antagonist oral anticoagulants (NOACs) might be superior to aspirin in preventing recurrent strokes after ESUS.
ESUS is a type of ischemic stroke with unknown origin, i.e. for which no probable cause can be identified after standard diagnostic evaluation. Thus, ESUS is a subgroup of cryptogenic stroke, which also includes strokes with incomplete evaluation and those for which more than one probable cause. Non-lacunar strokes in patients without no major-risk cardioembolic source (such as atrial fibrillation), no major atherosclerosis of the arteries supplying the brain infarct area and no other specific cause of their stroke are identified as ESUS.
Current strategy for secondary stroke prevention is based on antiplatelets but stroke recurrent rates remain high. A historical trial, WARSS, pointed at a potential advantage of anticoagulation over antiplatelet in patients with cryptogenic stroke. There is broad evidence for anticoagulation for stroke prevention in atrial fibrillation, thus providing another rationale for testing anticoagulation in ESUS.
Two large trials randomized ESUS patients for long-term treatment with either a NOAC or aspirin: NAVIGATE ESUS was stopped after an interim analysis as efficacy was not different with rivaroxaban compared with aspirin but showed an increased risk of major bleeding. RE-SPECT ESUS is still ongoing as planned; in this trial dabigatran is tested against aspirin. Results will be presented in October 2018. If the trial is positive, i.e. showing greater efficacy of dabigatran in the prevention of stroke recurrence than aspirin, this may not only have great implications of how ESUS patients are treated but could also lead to a simplified post-stroke diagnostic workup for most patients with ischemic stroke.
Please click on the photo below to access the round table video.








 Decompressive craniectomy (removal of parts of the cranial bone) is a standard surgical treatment, which is beneficial in patients with brain swelling after a severe ischemic stroke (under- supply of blood and oxygen in certain brain regions due to occlusion of a blood vessel by a blood clot), after brain injuries, but also in patients with meningitis. However, no trial has so far investigated the effectiveness of decompressive craniectomy in patients with brain bleeding. With this trial we would like to investigate in patients with a brain bleeding, whether this treatment method can reduce mortality and dependency compared to best medical treatment. In a larger context, this trial aims to offer a future treatment option to patients with brain bleeding, which can reduce both mortality and disability.
Decompressive craniectomy (removal of parts of the cranial bone) is a standard surgical treatment, which is beneficial in patients with brain swelling after a severe ischemic stroke (under- supply of blood and oxygen in certain brain regions due to occlusion of a blood vessel by a blood clot), after brain injuries, but also in patients with meningitis. However, no trial has so far investigated the effectiveness of decompressive craniectomy in patients with brain bleeding. With this trial we would like to investigate in patients with a brain bleeding, whether this treatment method can reduce mortality and dependency compared to best medical treatment. In a larger context, this trial aims to offer a future treatment option to patients with brain bleeding, which can reduce both mortality and disability.











