Swiss trial of decompressive craniectomy versus best medical treatment of spontaneous supratentorial intracerebral hemorrhage (SWITCH): a randomized controlled trial
Background
The SWITCH trial investigates the treatment of patients with spontaneous intracerebral hemorrhage (bleeding in the brain). Bleeding in the brain leads to severe brain dysfunctions due to the hemorrhage itself, but also from the brain swelling (brain edema). Each year, about 2 million people worldwide are affected by this disease. The majority of surviving patients remains handicapped. Apart from the standard best medical treatment, there is no possibility to help these patients until now.
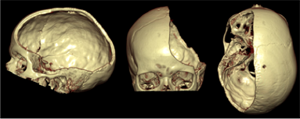 Decompressive craniectomy (removal of parts of the cranial bone) is a standard surgical treatment, which is beneficial in patients with brain swelling after a severe ischemic stroke (under- supply of blood and oxygen in certain brain regions due to occlusion of a blood vessel by a blood clot), after brain injuries, but also in patients with meningitis. However, no trial has so far investigated the effectiveness of decompressive craniectomy in patients with brain bleeding. With this trial we would like to investigate in patients with a brain bleeding, whether this treatment method can reduce mortality and dependency compared to best medical treatment. In a larger context, this trial aims to offer a future treatment option to patients with brain bleeding, which can reduce both mortality and disability.
Decompressive craniectomy (removal of parts of the cranial bone) is a standard surgical treatment, which is beneficial in patients with brain swelling after a severe ischemic stroke (under- supply of blood and oxygen in certain brain regions due to occlusion of a blood vessel by a blood clot), after brain injuries, but also in patients with meningitis. However, no trial has so far investigated the effectiveness of decompressive craniectomy in patients with brain bleeding. With this trial we would like to investigate in patients with a brain bleeding, whether this treatment method can reduce mortality and dependency compared to best medical treatment. In a larger context, this trial aims to offer a future treatment option to patients with brain bleeding, which can reduce both mortality and disability.
This project is organized by the Neuro Clinical Trial Unit at the University of Bern, Switzerland, and is carried out throughout Europe.
What does it mean for patients to participate in this clinical trial?
All patients will be will be assigned by chance to two groups (half of patients will be in each group). In the ‘treatment group’, surgery (decompressive craniectomy) plus best medical treatment will be performed. The patient’s own cranial bone will be re-inserted (re-implanted) after reduction of the swelling in the brain. This usually takes place after about 3 months. In the ‘standard group’, the participants receive best medical treatment, which is the current standard treatment.
A telephone interview will be performed with all patients 30 days, 6 and 12 months after randomization. Apart from the telephone interviews, all examinations are carried out regardless whether or not the patient participates in this trial.
General information about the trial
| Study type: |
Multicenter randomized (1:1) controlled parallel group trial
|
| Trial start and end: |
October 2014 to September 2020
|
| Sponsor-Investigator: |
Prof. Dr. med. Urs Fischer, Inselspital Bern, Switzerland
|
| Participant countries: |
Switzerland, Austria, Germany, Helsinki, Spain, the Netherlands, France
|
| Upcoming participant countries: |
UK
|
| Total Number of participants: | 300 patients |
| Trial duration for each participant: | 12 months |
| Financial support: | Swiss National Science Foundation (SNCF), Swiss Heart Foundation, Inselspital Foundation |
| Trial registration: | www.clinicaltrials.gov, No.: NCT02258919 |
For further information, please visit the SWITCH Website
This project is endorsed by SAFE.





