
Jul 27, 2018
The 15 min round table discussion by Christina Sjöstrand, Stockholm, Hans-Christoph Diener, Essen and George Ntaios, Thessaly, focuses on the concepts of randomized clinical trials for secondary stroke prevention in patients with embolic stroke of undetermined source (ESUS). Two large clinical trials tested the hypothesis that non-vitamin K antagonist oral anticoagulants (NOACs) might be superior to aspirin in preventing recurrent strokes after ESUS.
ESUS is a type of ischemic stroke with unknown origin, i.e. for which no probable cause can be identified after standard diagnostic evaluation. Thus, ESUS is a subgroup of cryptogenic stroke, which also includes strokes with incomplete evaluation and those for which more than one probable cause. Non-lacunar strokes in patients without no major-risk cardioembolic source (such as atrial fibrillation), no major atherosclerosis of the arteries supplying the brain infarct area and no other specific cause of their stroke are identified as ESUS.
Current strategy for secondary stroke prevention is based on antiplatelets but stroke recurrent rates remain high. A historical trial, WARSS, pointed at a potential advantage of anticoagulation over antiplatelet in patients with cryptogenic stroke. There is broad evidence for anticoagulation for stroke prevention in atrial fibrillation, thus providing another rationale for testing anticoagulation in ESUS.
Two large trials randomized ESUS patients for long-term treatment with either a NOAC or aspirin: NAVIGATE ESUS was stopped after an interim analysis as efficacy was not different with rivaroxaban compared with aspirin but showed an increased risk of major bleeding. RE-SPECT ESUS is still ongoing as planned; in this trial dabigatran is tested against aspirin. Results will be presented in October 2018. If the trial is positive, i.e. showing greater efficacy of dabigatran in the prevention of stroke recurrence than aspirin, this may not only have great implications of how ESUS patients are treated but could also lead to a simplified post-stroke diagnostic workup for most patients with ischemic stroke.
Please click on the photo below to access the round table video.

Jul 25, 2018
Every year, up to 1.3 million people in Europe suffer a first stroke. Acute stroke treatment strategies such as acute treatment of patients in a stroke unit, intravenous thrombolysis and endovascular treatment significantly improve the outcome for patients with ischaemic stroke and thus reduce its socioeconomic burden. However, reliable data on access to and delivery of acute stroke treatment strategies throughout Europe are lacking.
The European Stroke Organisation (ESO), the European Society of Minimally Invasive Neurological Therapy (ESMINT), the European Academy of Neurology (EAN) and the patient organisation Stroke Alliance for Europe (SAFE) have therefore surveyed stroke experts from 44 of 51 European countries on the best available information regarding national access to and delivery rates of acute stroke unit care, intravenous thrombolysis, and endovascular treatment.
The results of this survey identify major inequalities in acute stroke treatment, with many countries reporting rates that were far below highest country rates. This article shows in which countries patients still have no or obviously inadequate access to appropriate acute stroke treatment.
According to Urs Fischer, Professor for Acute Neurology and Stroke at the University Hospital Bern, up to 226,662 additional patients could be treated with intravenous thrombolysis and an additional 67,347 with endovascular treatment each year. “Many stroke victims in Europe still have no access to acute stroke treatment,” he said. “If stroke unit care, intravenous thrombolysis and endovascular therapy would be routine practice throughout Europe, many more victims could survive their stroke without a major handicap”. According to Prof. Fischer, these inequalities not only have a major impact on the patients, but also on their families and the socioeconomic burden. “Recent studies have shown, that acute stroke treatment is highly cost effective – if you treat your patients right, you will not only improve the quality of life of the patient and his family, you will also save money for your society”, he said. Therefore, efforts have to be done to increase the number of stroke units, intravenous thrombolysis and endovascular stroke therapy. “We have to talk to governments, politicians, health care specialists and stroke physicians in order to set up and improve the system in regions, where acute stroke treatment is lacking!”
In May 2018, ESO and SAFE presented the Stroke Action Plan for Europe to set achievement goals for stroke care by 2030. This survey already provided important insights for the Stroke Action Plan authors. The findings from this survey are relevant for future health-care planning in Europe and beyond.
Contact
Prof. Dr. med Urs Fischer
Department of Neurology, University Hospital Bern
urs.fischer@insel.ch European Stroke Organisation www.eso-stroke.org
Article Title and Citation
Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries
http://journals.sagepub.com/doi/full/10.1177/2396987318786023
Authors
Diana Aguiar de Sousa, Rascha von Martial, Sònia Abilleira, Thomas Gattringer, Adam Kobayashi, Miquel Gallofré, Franz Fazekas, Istvan Szikora, Valery Feigin, Valeria Caso, Urs Fischer, on behalf of the ESO ESMINT EAN SAFE Survey on Stroke Care collaborators
About the Authors
Diana Aguiar de Sousa: First author
Diana Aguiar de Sousa is Neurologist at Hospital de Santa Maria, in Lisbon, and assistant professor of Neuroanatomy at University of Lisbon. She is clinical investigator at Instituto de Medicina Molecular and PhD student at University of Lisbon, under the guidance of Prof. JM Ferro and Prof. P. Canhão. She completed the Clinical Scholars Research Training Certificate Program from Harvard Medical School and a European Academy of Neurology Research Fellowship in acute stroke at Inselspital Bern, Switzerland. Diana Sousa was awarded with the European Stroke Organization (ESO) Young Investigator Award in 2016. She is member of the ESO Young Stroke Physicians and Researchers Committee.
Urs Fischer: Corresponding author
Urs Fischer (urs.fischer@insel.ch)is Professor for Acute Neurology and Stroke (Extraordinarius) at the University Hospital (Inselspital), Bern. He is chair of the Neurological Inpatient Department, co-chair of the Stroke Center Bern, and deputy director of the Clinical Trial Unit (CTU) of the University of Bern. He is a clinical researcher and his main research interest involves diagnosis, treatment and outcome of patients with acute neurological diseases. He is co-principal investigator of the SWITCH study (www.switch-trial.ch), principal investigator of the ELAN trial (www.elan-trial.ch) and co-principal investigator of the SWIFT DIRECT trial (www.swift-direct.ch). Urs Fischer is Secretary General of the European Stroke Organisation (ESO), treasurer of the Swiss Neurological Society (SNG) and co-founder the ESO ESMINT ESNR Stroke Winter School, which is regularly held at the University Hospital in Bern, Switzerland.

Jul 24, 2018
Since this May, in selected hospitals in 12 European countries: Spain, Serbia, Poland, Czech Republic, Latvia, Croatia, Macedonia, Greece, Ukraine, Georgia, Hungary and Turkey, stroke patients and their carers are being provided with information on stroke and important next steps on their path to recovery.
The patient-focused materials are made of five brochures, including a list of national, regional and local stroke support organisations, with their contact details, in order that patients and carers can access further support in the months and years following their stroke. The information provided in the brochures are kindly provided by the Stroke Association UK and then translated to all project languages, applying the information standard procedure for the translation.
The next step in SAFE Angels project consists of collecting feedback from patients and their carers about the quantity and quality of information they have been provided with during their stay at the hospital, before being discharged. The feedback will be collected through an online survey in all 13 project languages (Spanish, Catalan, Serbian, Polish, Czech, Latvian, Croatian, Macedonian, Greek, Ukrainian, Georgian, Hungarian and Turkish).
Please see the survey on this link and inform the people you know.
For more information about the hospitals which are providing patient materials, please find an organisation which is working in your country here.
If you are interested in finding out more about the SAFE Angels project, please contact Jelena Misita, SAFE Awareness and Advocacy Manager at jelena.misita@safestroke.eu.
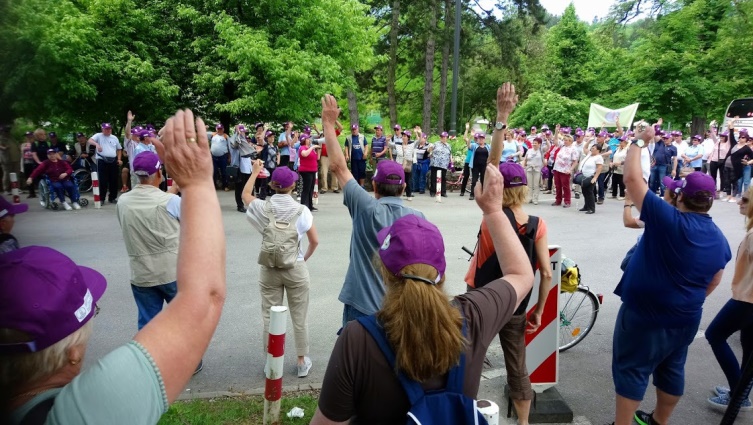
Jul 23, 2018
Slovenian stroke support organisation »Združenje bolnikov s cerebrovaskularno boleznijo Slovenije« is one of SAFE’s very active members. We recently received an authored text about their stroke awareness activities in May 2018 from Jelka Janša, who is an active member of the Slovenian stroke support organisation and past SAFE President.
…
Written by Jelka Janša
 To promote the European Stroke day, there were two main activities namely: the city walk in Ljubljana and two teleconferences. In addition, 400 posters with message about FAST campaign in Slovenian – GROM were displayed around Slovenia for a period of April 24 till May 8. Further to that, 8 professional lectures were sponsored to be delivered within stroke clubs around Slovenia aiming to share again information about the facts about stroke.
To promote the European Stroke day, there were two main activities namely: the city walk in Ljubljana and two teleconferences. In addition, 400 posters with message about FAST campaign in Slovenian – GROM were displayed around Slovenia for a period of April 24 till May 8. Further to that, 8 professional lectures were sponsored to be delivered within stroke clubs around Slovenia aiming to share again information about the facts about stroke.
 Second city walk in Ljubljana was on actual European Stroke Day, May 8. This year the distance was 1,6 km and there were 260 stroke survivors and their supporters attending. It ended at the city Centre, »Prešernov trg«, were there were stands with information about stroke, its prevention including leaflets about Slovenian Stroke Support Organisation and Slovenian Heart Organisation. People, passing by, had the opportunity to have their cholesterol and blood pressure checked. There were four speeches, by Milan Čuček, president of SSSO, Tatjana Erjavec, vice-president of the SSSO, Mr. Zalar from Heart organisation and by Mr Jankovič, the major of Ljubljana. Participants continued with a lunch at the Livada restaurant in Ljubljana.
Second city walk in Ljubljana was on actual European Stroke Day, May 8. This year the distance was 1,6 km and there were 260 stroke survivors and their supporters attending. It ended at the city Centre, »Prešernov trg«, were there were stands with information about stroke, its prevention including leaflets about Slovenian Stroke Support Organisation and Slovenian Heart Organisation. People, passing by, had the opportunity to have their cholesterol and blood pressure checked. There were four speeches, by Milan Čuček, president of SSSO, Tatjana Erjavec, vice-president of the SSSO, Mr. Zalar from Heart organisation and by Mr Jankovič, the major of Ljubljana. Participants continued with a lunch at the Livada restaurant in Ljubljana.
Two teleconferences were held to emphasize the message about Life after stroke and stroke facts; the first one already on April 23 and organized by Slovenian Heart organisation with representatives from SSSO. They were talking about stroke issues and notified the journalist already about the ESD and the next teleconference, on May 23. The May’s conference was organized by the SSSO and in addition to talking about the life after stroke issues, Tatjana Erjavec also spoke about »Stroke Action Plan in Europe«, the policy paper prepared by both the SAFE and ESO and presented at the same day in EP.
May activities have been well covered by national and local media with 66 publications, including TV, radio and newspapers.The activities were kindly sponsored by University rehabilitation Institute –SOČA, Bayer, Boehringer Ingelheim, Medis, Soča Oprema, Timing Ljubljana, SERVIER Pharma and Mountain Rescue team.
Please look at some of the photos from these activities.






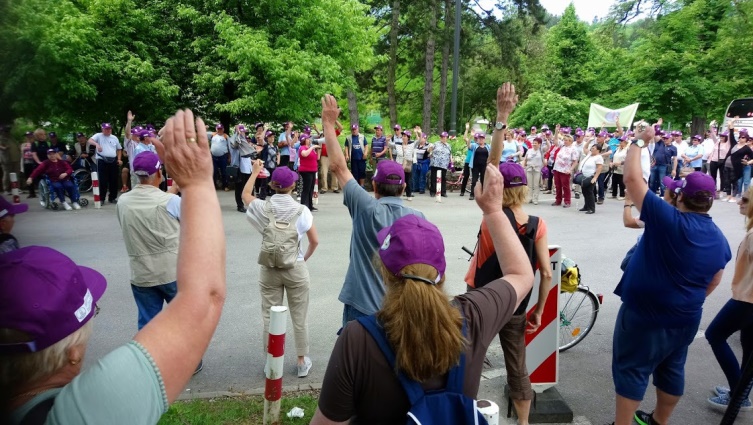

Jul 20, 2018
The World Stroke Congress 2018’s late breaking abstract submission is now open. Don’t miss this last opportunity to be part of this year’s program! Share your work now, and get valuable feedback from colleagues and leaders in the field, opportunities for collaborations and ideas for future research. Review the topics and the guidelines, and submit your abstract today.
The deadline for submission is August 15. Find all the information here.






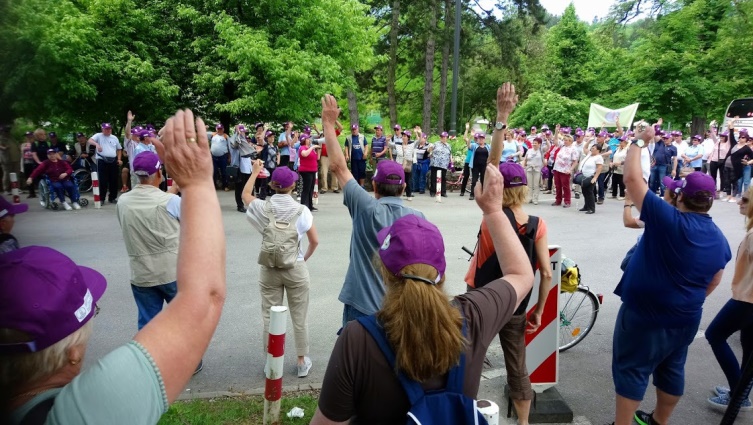
 To promote the European Stroke day, there were two main activities namely: the city walk in Ljubljana and two teleconferences. In addition, 400 posters with message about FAST campaign in Slovenian – GROM were displayed around Slovenia for a period of April 24 till May 8. Further to that, 8 professional lectures were sponsored to be delivered within stroke clubs around Slovenia aiming to share again information about the facts about stroke.
To promote the European Stroke day, there were two main activities namely: the city walk in Ljubljana and two teleconferences. In addition, 400 posters with message about FAST campaign in Slovenian – GROM were displayed around Slovenia for a period of April 24 till May 8. Further to that, 8 professional lectures were sponsored to be delivered within stroke clubs around Slovenia aiming to share again information about the facts about stroke. Second city walk in Ljubljana was on actual European Stroke Day, May 8. This year the distance was 1,6 km and there were 260 stroke survivors and their supporters attending. It ended at the city Centre, »Prešernov trg«, were there were stands with information about stroke, its prevention including leaflets about Slovenian Stroke Support Organisation and Slovenian Heart Organisation. People, passing by, had the opportunity to have their cholesterol and blood pressure checked. There were four speeches, by Milan Čuček, president of SSSO, Tatjana Erjavec, vice-president of the SSSO, Mr. Zalar from Heart organisation and by Mr Jankovič, the major of Ljubljana. Participants continued with a lunch at the Livada restaurant in Ljubljana.
Second city walk in Ljubljana was on actual European Stroke Day, May 8. This year the distance was 1,6 km and there were 260 stroke survivors and their supporters attending. It ended at the city Centre, »Prešernov trg«, were there were stands with information about stroke, its prevention including leaflets about Slovenian Stroke Support Organisation and Slovenian Heart Organisation. People, passing by, had the opportunity to have their cholesterol and blood pressure checked. There were four speeches, by Milan Čuček, president of SSSO, Tatjana Erjavec, vice-president of the SSSO, Mr. Zalar from Heart organisation and by Mr Jankovič, the major of Ljubljana. Participants continued with a lunch at the Livada restaurant in Ljubljana.











