
Aug 28, 2018
The original information provided at Braincouncil.eu
In July this year, European Brain Council launched a statement “Counting down to zero: Towards a future with underfunded health research?” which calls on the European Commission, the European Parliament and the Council to increase the budget of the “Horizon Europe” programme to at least €120 billion. What is more, the statement calls on European decision-makers to redistribute the budget in order to ensure that more funding is allocated to the “health” cluster under Pillar II.
The proposal for “Horizon Europe”, published in June 2018, includes an overall budget of €94.1 billion in inflation-adjusted prices. Within this budget, roughly €7.7 billion is proposed to fund the “Health” cluster that is part of the pillar “Global Challenges and Industrial Competitiveness”.
EBC welcomes the increase of the overall draft budget but firmly believes that the proposed budget as it currently stands is insufficient to effectively address today’s societal challenges. The treatment of brain disorders alone is estimated to cost close to €800 billion annually[1], which makes adopting a robust 9th Framework Programme with a strong focus on accelerating health research of paramount importance. What is more, the proposed budget for the “Health” cluster confirms a steady decrease of funding over time and across Framework Programmes, as health was previously allocated 12% under the 7th Framework Programme, 10% under Horizon 2020 and now 8% in the Horizon Europe proposal.
“We are highly concerned about the budget of €7.7 billion provisionally allocated to the “health” cluster under
Pillar II. This amount is not commensurate with the total budget increase and will clearly be insufficient to
effectively address the societal challenges associated with health research. Moreover, this budget confirms a
steady decrease of funding over time and across Framework Programmes, as health was previously allocated
12% under the 7th Framework Programme, 10% under Horizon 2020 and now 8% in the Horizon Europe
proposal. For continued success in European research, we find it imperative that this downward trend is
stopped and reverted.”– says the statement.
The full statement can be accessed here.
EBC invites all organizations, operating at national and/or EU level, that are supportive of the call to join as co-signatory of the statement. Please contact the EBC office (info@braincouncil.eu or +32 (0)2 513 27 57) should you be interested in having the logo of your organization added to the list of signatories.


Aug 24, 2018
The following content was first published on EU Commission official website
According to a recently published study, European patients are still generally unaware of their rights and the possibility to access health services in other EU Member States, as well as of the existence of National Contact Points (NCPs). But the situation is improving.
National Contact Points (NCPs) aim to help patients exercise their rights under the Cross-border Healthcare Directive. But how can they improve their work?
Using a combination of research methods, including a literature review, an analysis of legal texts, a website analysis, a pseudo-patient investigation, and surveys of NCPs and patients, the aim of the study carried out by Ecorys, KU Leuven and GfK Belgium was to identify how to improve the current level of information on cross-border healthcare available to patients.
Websites
The study found that although the information available to patients on NCP websites was adequate, the websites themselves need improvements, especially the sections on patients’ rights (for incoming patients), quality and safety standards (for incoming patients) and reimbursement of cross-border healthcare costs (for outgoing patients).
However, compared to the results of the earlier Evaluative study(fieldwork carried out in 2014), the NCPs have made significant progress in this area.
Toolbox and training material
This study has also resulted in the development of a practice-orientated toolbox and training material to help the NCPs improve the quality of information for patients, as well as a set of Guiding Principles and indicators for establishing an NCP service that is more uniform, patient-centred and in line with the legal requirements. This will contribute to high level information provision to patients.
The study feeds into the upcoming implementation report on the operation of the Cross-border Healthcare Directive due this October.
More information

Aug 8, 2018
Solitaire™ With the Intention For Thrombectomy Plus Intravenous t-PA Versus DIRECT Solitaire™ Stent-retriever Thrombectomy in Acute Anterior Circulation Stroke (SWIFT DIRECT)
Background
The SWIFT DIRECT trial investigates the emergency treatment of patients with an acute ischaemic stroke. An ischaemic stroke is caused by the blockage of one or more blood vessels in the brain. A clot blocks the blood vessels and blood can no longer circulate. This results in an undersupply of blood and oxygen to the brain regions supplied by these vessels. If the under-supply lasts longer than a few minutes, there is a risk that nerve cells might die. An ischaemic cerebral infarction is a life-threatening situation.
For years, the only causal treatment was the administration of a clot-lysing drug called tissue plasminogen activator. The drug, however, may induce bleedings and is not sufficiently effective in patients with very large clots. In 2014, a new treatment, the so-called mechanical thrombectomy, has been established. With this therapy, nearly all types of large blood clots can directly be removed from the vessel using a specialized catheter. Trials have shown that patients treated with tissue plasminogen activator and mechanical thrombectomy have better outcomes than patients treated with tissue plasminogen activator only. Hence, the current standard treatment in patients with large clots is administration of tissue plasminogen activator followed by mechanical thrombectomy.
As administration of tissue plasminogen activator may also harm the patient and is not effective in patients with large clots, we want to investigate how potent direct mechanical thrombectomy (without prior administration of tissue plasminogen activator) is. The purpose of this trial is thus to compare direct removal of the clot with mechanical thrombectomy versus tissue plasminogen activator administration followed by removal of the clot with mechanical thrombectomy. Only patients with large clots and direct access to mechanical thrombectomy can be included in the trial.
We are conducting this trial to improve the emergency treatment for affected patients with an acute ischaemic stroke. This project is organised by the Neuro Clinical Trial Unit at the University of Bern, Switzerland and will be carried out at several hospitals in Europe and Canada.
What does it mean for patients to participate in this clinical trial?
Trial participants will be assigned by chance to one of two groups (half of the patients will be in each group). In the ‘treatment group’ the blood clot is removed directly with mechanical thrombectomy. In the ‘standard group’, participants first receive blood clot-dissolving medication followed by mechanical thrombectomy to remove the clot.
Both treatment options are commonly used standard treatments. The choice between the two is part of clinical routine at the hospital and lies upon the judgement of the treating physician. Except for the phone interview 90 days after the infarction incident, all examinations are part of standard treatment routine independent from the trial.
General information about the trial
| Study type: |
Multicenter, prospective, randomized, open label, blinded endpoint (PROBE) trial |
| Trial start and end: |
October 2017 to December 2020 |
| Sponsor-Investigators: |
Prof. Dr. med. Urs Fischer, Neurology, and
Prof. Dr. med. Jan Gralla, Neuroradiology,
University Hospital Bern, Inselspital, Switzerland |
| Total number of participants: |
404 patients |
| Trial duration for each participant: |
3 months |
| Participating countries: |
Austria, Canada, Finland, France, Germany, Spain and Switzerland |
| Financial support: |
Medtronic (Minneapolis, USA) |
| Trial registration: |
www.clinicaltrials.gov, No.: NCT03192332 |
Please visit the website www.swift-direct.com for further information.
This trial is endorsed by SAFE.

Aug 8, 2018
Swiss trial of decompressive craniectomy versus best medical treatment of spontaneous supratentorial intracerebral hemorrhage (SWITCH): a randomized controlled trial
Background
The SWITCH trial investigates the treatment of patients with spontaneous intracerebral hemorrhage (bleeding in the brain). Bleeding in the brain leads to severe brain dysfunctions due to the hemorrhage itself, but also from the brain swelling (brain edema). Each year, about 2 million people worldwide are affected by this disease. The majority of surviving patients remains handicapped. Apart from the standard best medical treatment, there is no possibility to help these patients until now.
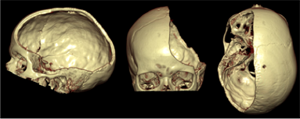 Decompressive craniectomy (removal of parts of the cranial bone) is a standard surgical treatment, which is beneficial in patients with brain swelling after a severe ischemic stroke (under- supply of blood and oxygen in certain brain regions due to occlusion of a blood vessel by a blood clot), after brain injuries, but also in patients with meningitis. However, no trial has so far investigated the effectiveness of decompressive craniectomy in patients with brain bleeding. With this trial we would like to investigate in patients with a brain bleeding, whether this treatment method can reduce mortality and dependency compared to best medical treatment. In a larger context, this trial aims to offer a future treatment option to patients with brain bleeding, which can reduce both mortality and disability.
Decompressive craniectomy (removal of parts of the cranial bone) is a standard surgical treatment, which is beneficial in patients with brain swelling after a severe ischemic stroke (under- supply of blood and oxygen in certain brain regions due to occlusion of a blood vessel by a blood clot), after brain injuries, but also in patients with meningitis. However, no trial has so far investigated the effectiveness of decompressive craniectomy in patients with brain bleeding. With this trial we would like to investigate in patients with a brain bleeding, whether this treatment method can reduce mortality and dependency compared to best medical treatment. In a larger context, this trial aims to offer a future treatment option to patients with brain bleeding, which can reduce both mortality and disability.
This project is organized by the Neuro Clinical Trial Unit at the University of Bern, Switzerland, and is carried out throughout Europe.
What does it mean for patients to participate in this clinical trial?
All patients will be will be assigned by chance to two groups (half of patients will be in each group). In the ‘treatment group’, surgery (decompressive craniectomy) plus best medical treatment will be performed. The patient’s own cranial bone will be re-inserted (re-implanted) after reduction of the swelling in the brain. This usually takes place after about 3 months. In the ‘standard group’, the participants receive best medical treatment, which is the current standard treatment.
A telephone interview will be performed with all patients 30 days, 6 and 12 months after randomization. Apart from the telephone interviews, all examinations are carried out regardless whether or not the patient participates in this trial.
General information about the trial
| Study type: |
Multicenter randomized (1:1) controlled parallel group trial
|
| Trial start and end: |
October 2014 to September 2020
|
| Sponsor-Investigator: |
Prof. Dr. med. Urs Fischer, Inselspital Bern, Switzerland
|
| Participant countries: |
Switzerland, Austria, Germany, Helsinki, Spain, the Netherlands, France
|
| Upcoming participant countries: |
UK
|
| Total Number of participants: |
300 patients |
| Trial duration for each participant: |
12 months |
| Financial support: |
Swiss National Science Foundation (SNCF), Swiss Heart Foundation, Inselspital Foundation |
| Trial registration: |
www.clinicaltrials.gov, No.: NCT02258919 |
For further information, please visit the SWITCH Website
This project is endorsed by SAFE.
Aug 6, 2018
Oruen – The CNS Journal is a peer-reviewed, open access publication, and has received CME accreditation from the European Accreditation Committee in CNS (EACIC), with a 100% focus on original CNS research topics, and the latest advances, diagnoses, and treatment of CNS disorders.
The Journal is distributed in print and electronically to thousands of physicians, researchers, academics, nurses, and related healthcare professionals with an interest in CNS disorders. Both subscription and access are free and there are no contributory author fees for publication. Papers submitted for publication are accepted based on their originality, likely impact on and relevance to clinical practice, data quality, and overall potential interest to the journal’s readership.
Oruen – The CNS Journal is published bi-annually. The first issue of the journal was published in May 2015
You can access the latest issue by clicking on the photo below:
For any questions or submission requests/enquiries please contact Dr James Coe – Head Editor editor@oruen.com






 Decompressive craniectomy (removal of parts of the cranial bone) is a standard surgical treatment, which is beneficial in patients with brain swelling after a severe ischemic stroke (under- supply of blood and oxygen in certain brain regions due to occlusion of a blood vessel by a blood clot), after brain injuries, but also in patients with meningitis. However, no trial has so far investigated the effectiveness of decompressive craniectomy in patients with brain bleeding. With this trial we would like to investigate in patients with a brain bleeding, whether this treatment method can reduce mortality and dependency compared to best medical treatment. In a larger context, this trial aims to offer a future treatment option to patients with brain bleeding, which can reduce both mortality and disability.
Decompressive craniectomy (removal of parts of the cranial bone) is a standard surgical treatment, which is beneficial in patients with brain swelling after a severe ischemic stroke (under- supply of blood and oxygen in certain brain regions due to occlusion of a blood vessel by a blood clot), after brain injuries, but also in patients with meningitis. However, no trial has so far investigated the effectiveness of decompressive craniectomy in patients with brain bleeding. With this trial we would like to investigate in patients with a brain bleeding, whether this treatment method can reduce mortality and dependency compared to best medical treatment. In a larger context, this trial aims to offer a future treatment option to patients with brain bleeding, which can reduce both mortality and disability.




