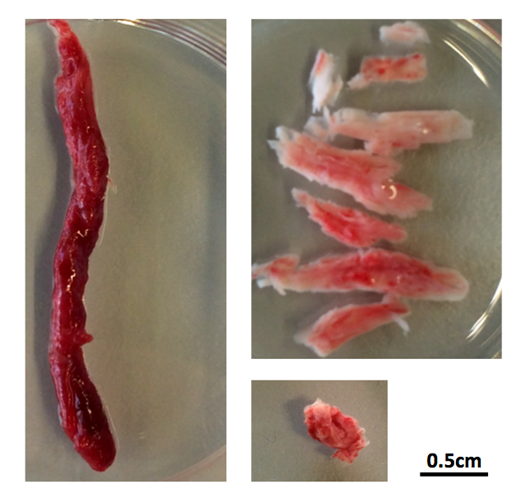
Critical differences in clots that cause a stroke
The original article was first published on ScienceDaily.com
There are two main treatments for stroke caused by a clot in a blood vessel in the brain. One treatment, mechanical thrombectomy, involves pulling the clot out with a specialized catheter that is inserted into the artery in the groin and guided by imaging to the clot. This procedure is only performed at hospitals that specialize in these techniques. The other treatment, which is more widely accessible, involves giving a patient a clot-busting drug that helps the body dissolve the clot.
Quick decision making on which treatment is best for which patient is critical because the clot deprives brain cells of oxygen causing them to die. For physicians, knowing which patients will benefit the most from the clot-buster Alteplase (also known as tPA) just got easier.
University of Calgary scientists with the Hotchkiss Brain Institute at the Cumming School of Medicine (CSM) have discovered that clots have different compositions and depending on where they are located in the brain, administering tPA can be almost as effective as thrombectomy given sufficient time.
“We’ve known that, when administered quickly, tPA can be effective in stroke, but until now, we didn’t realize how effective it can be and we didn’t understand the specific reasons why it works better in some cases than others,” says Dr. Bijoy Menon, MD, associate professor in the departments of Clinical Neurosciences, Radiology and Community Health Sciences at the CSM. “Our findings show that some clots are permeable, which allows the tPA to penetrate the blockage and dissolve it. We saw that within two hours, greater than 50 per cent of permeable blockages had dissolved.”
The UCalgary study led out of the Foothills Medical Centre is the largest of its kind to date, involving nearly 600 patients at 12 medical centres in five countries (Canada, the Czech Republic, South Korea, Spain and Turkey). The findings are published in JAMA.
“Despite earlier research on the benefit of using tPA, we know there is still some reluctance in the medical community to use it. These findings should provide physicians with definitive evidence on the value of giving patients tPA as soon as they’ve confirmed the stroke is due to a clot,” says Dr. Andrew Demchuk, MD, professor in the departments of Clinical Neurosciences and Radiology. “It’s critical that anyone showing symptoms of a stroke be given a CT-angiogram as soon as possible to confirm the blockage. The scan will guide whether tPA is likely to dissolve the clot and may inform whether the patient also needs thrombectomy.”
A CT-angiogram (computer tomography scan) is a common noninvasive diagnostic tool that allows physicians to see images of the blood vessels in the brain. Researchers found that clots in the carotid artery of the brain do not respond to tPA, and for these patients, thrombectomy is required.
“Strokes happen at anytime, anywhere. Knowing who needs thrombectomy can help physicians make better decisions on how to prioritize patient transfers to specialized centres for this procedure,” says Menon. “Data gathered in Europe showed that up to one-third of hospital transfers aren’t necessary.”
“Stroke is an important health care problem and one of the leading causes of death and disability worldwide,” says Dr. Brian H. Rowe, scientific director, Canadian Institutes of Health Research (CIHR) Institute of Circulatory and Respiratory Health, which supported this study. “Through continued scientific research, important discoveries like this one will improve our ability to match patients with the most effective treatment for this particular injury. This will help speed up recovery times, reduce the associated impacts such as paralysis, and it will improve patient outcomes and ultimately save lives.”
Drs. Menon and Demchuk add that for the science community these findings will help researchers better design studies that target dissolving the clot with new clot busting drugs or combination treatments.
Led by the Hotchkiss Brain Institute, Brain and Mental Health is one of six strategic research themes guiding the university towards its Eyes High goals. The strategy provides a unifying direction for brain and mental health research at the university and positions researchers to unlock new discoveries and treatments for brain health in our community.
Story Source: University of Calgary. “Critical differences in clots that cause a stroke: Findings will help inform physicians which treatment will work best for patients.” ScienceDaily. ScienceDaily, 12 September 2018. <www.sciencedaily.com/releases/2018/09/180912081219.htm>.




 Following the publication of EPF’s Transparency Guidelines, European Patients Forum would like to invite you to join a webinar to discuss transparent advocacy in patient organisations.
Following the publication of EPF’s Transparency Guidelines, European Patients Forum would like to invite you to join a webinar to discuss transparent advocacy in patient organisations.